WHAMM, a member of the Wiskott-Aldrich syndrome protein (WASP) family, is an actin nucleation promoting factor (NPF) that also associates with membranes and microtubules. Here we report that WHAMM is required for autophagic lysosome reformation (ALR). WHAMM knockout causes impairment of autolysosome tubulation, which results in accumulation of enlarged autolysosomes during prolonged starvation. Mechanistically, WHAMM is recruited to the autolysosome membrane through its specific interaction with PI(4,5)P2. WHAMM then works as an NPF which promotes assembly of an actin scaffold on the surface of the autolysosome to promote autolysosome tubulation. Our study demonstrates an unexpected role of the actin scaffold in regulating autophagic lysosome reformation.
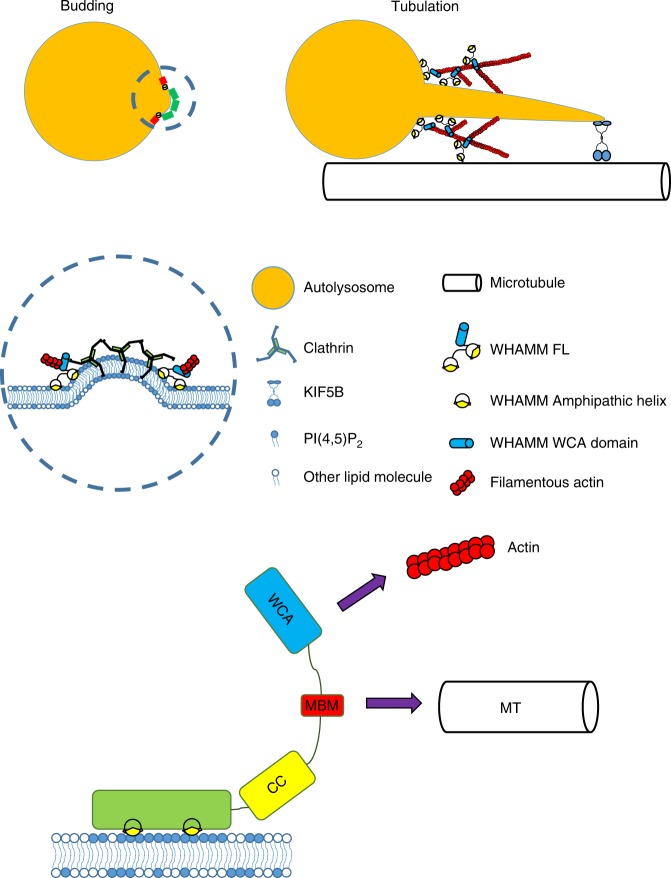
Dai A, Yu L*, Wang HW*. (2019) WHAMM initiates autolysosome tubulation by promoting actin polymerization on autolysosomes. Nat. Commun., 10(1): 3699. (*co-corresponding authors)
