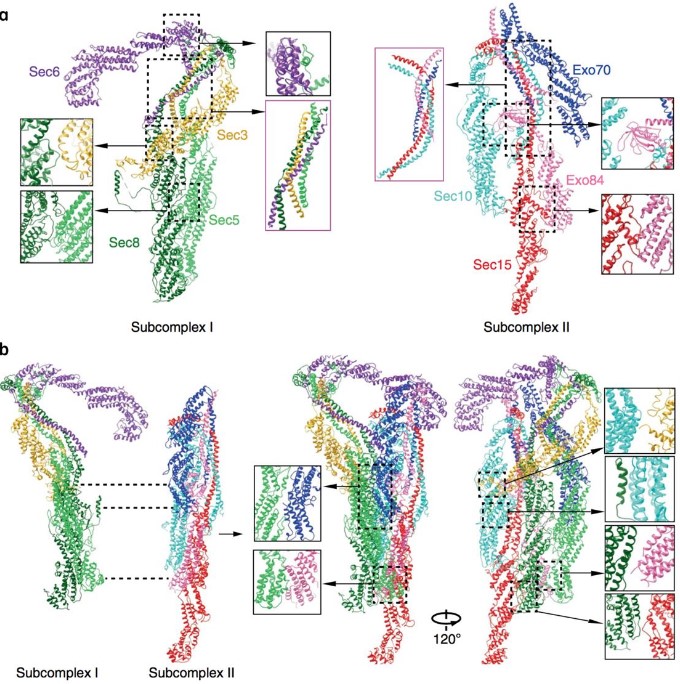
In eukaryotic cells, orderly transport, docking and fusion of intracellular vesicles to their cognate target membrane form elaborate vesicular trafficking network. Elucidating the structure and assembly of the multisubunit tethering complexes (MTCs) which initially tether vesicles and their target membrane is essential to the understanding of the mechanisms of vesicle tethering and fusion. Using cryo-EM and chemical cross-linking mass spectrum (CXMS), we solved the first intact structure of the Saccharomyces Cerevisiae exocyst complex, which functions in the tethering of secretory vesicles to the plasma membrane, at an average resolution of 4.4 Å. The model reveals insights into the hierarchical assembly of the exocyst complex that is mainly mediated by the assembly of the newly-defined CorEx motifs. Additional cell biological data indicates that Sec3 CorEx motif plays a critical role in the recruitment of other exocyst subunits and the tethering of secretory vesicles. Our work may shed light to the assembly and function mechanism of other MTCs, which will ultimately help elucidate the molecular mechanisms of vesicular trafficking.
External Links
Internal Links
-
News
- Cao et al. Structural basis of endo-siRNA processing by Drosophila Dicer-2 and Loqs-PD, Nucleic Acids Research, 2025
- Zheng et al. Cryo-electron tomography reconstructs polymer in liquid film for fab-compatible lithography, Nature Communications, 2025
- Zheng et al. Self-assembled superstructure alleviates air-water interface effect in cryo-EM, Nature Communications, 2024
- Yang et al. Electrospray-assisted cryo-EM sample preparation to mitigate interfacial effects, Nature Methods, 2024
- Xu et al. Graphene sandwich-based biological specimen preparation for cryo-EM analysis, PNAS, 2024