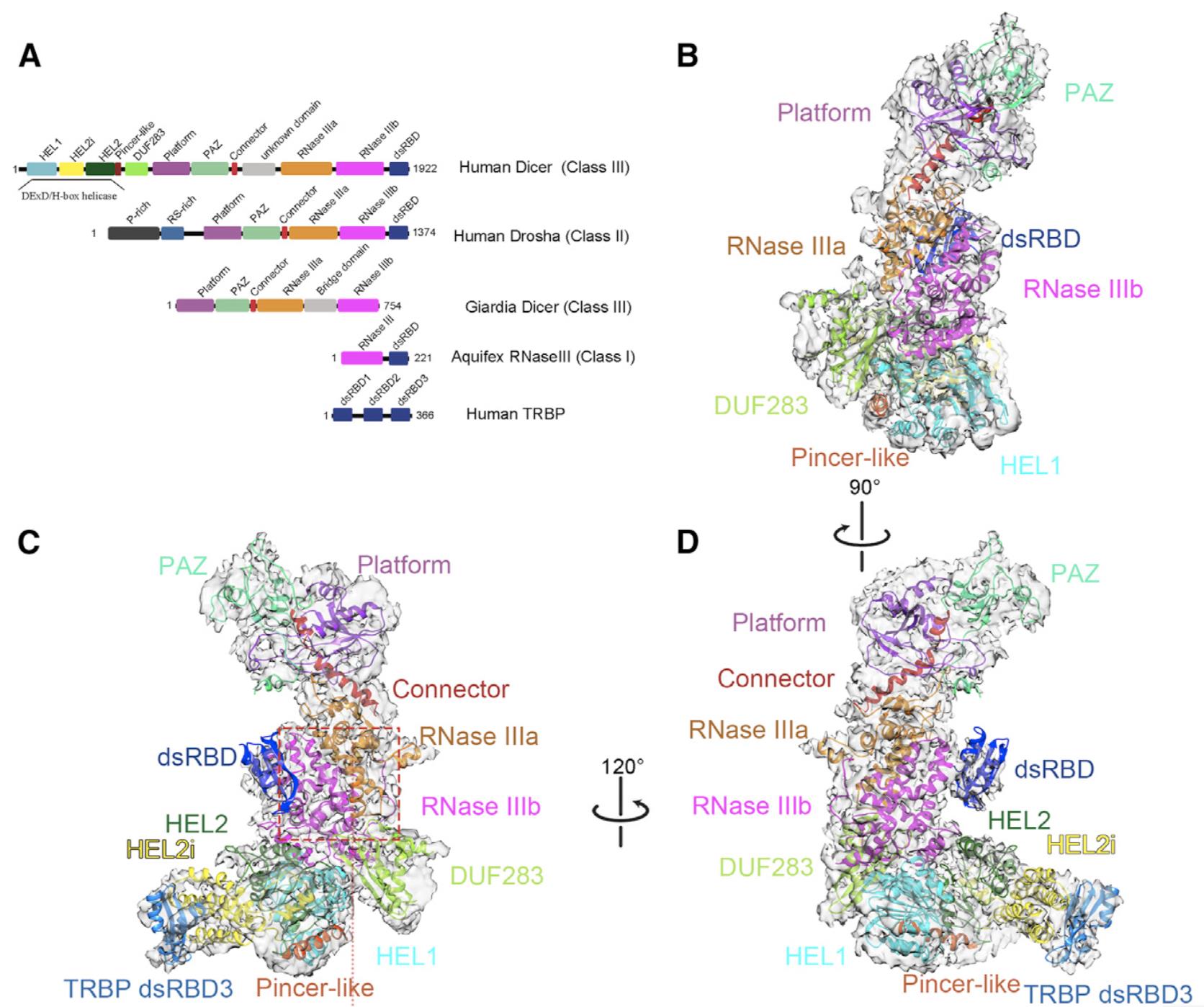
We have been studying the structure and mechanism of RNA exosome complex using single particle cryo-EM in the past ten years. We solved the cryo-EM structure of hDicer in complex with its cofactor protein TRBP and revealed the precise spatial arrangement of hDicer’s multiple domains. We further solved structures of the hDicer-TRBP complex bound with pre-let-7 RNA in two distinct conformations. In combination with biochemical analysis, these structures reveal a property of the hDicer-TRBP complex to promote the stability of pre-miRNA’s stem duplex in a predicting state. These results provide insights into the mechanism of RNA processing by hDicer and illustrate the regulatory role of hDicer’s N-terminal helicase domain.
External Links
Internal Links
-
News
- Cao et al. Structural basis of endo-siRNA processing by Drosophila Dicer-2 and Loqs-PD, Nucleic Acids Research, 2025
- Zheng et al. Cryo-electron tomography reconstructs polymer in liquid film for fab-compatible lithography, Nature Communications, 2025
- Zheng et al. Self-assembled superstructure alleviates air-water interface effect in cryo-EM, Nature Communications, 2024
- Yang et al. Electrospray-assisted cryo-EM sample preparation to mitigate interfacial effects, Nature Methods, 2024
- Xu et al. Graphene sandwich-based biological specimen preparation for cryo-EM analysis, PNAS, 2024