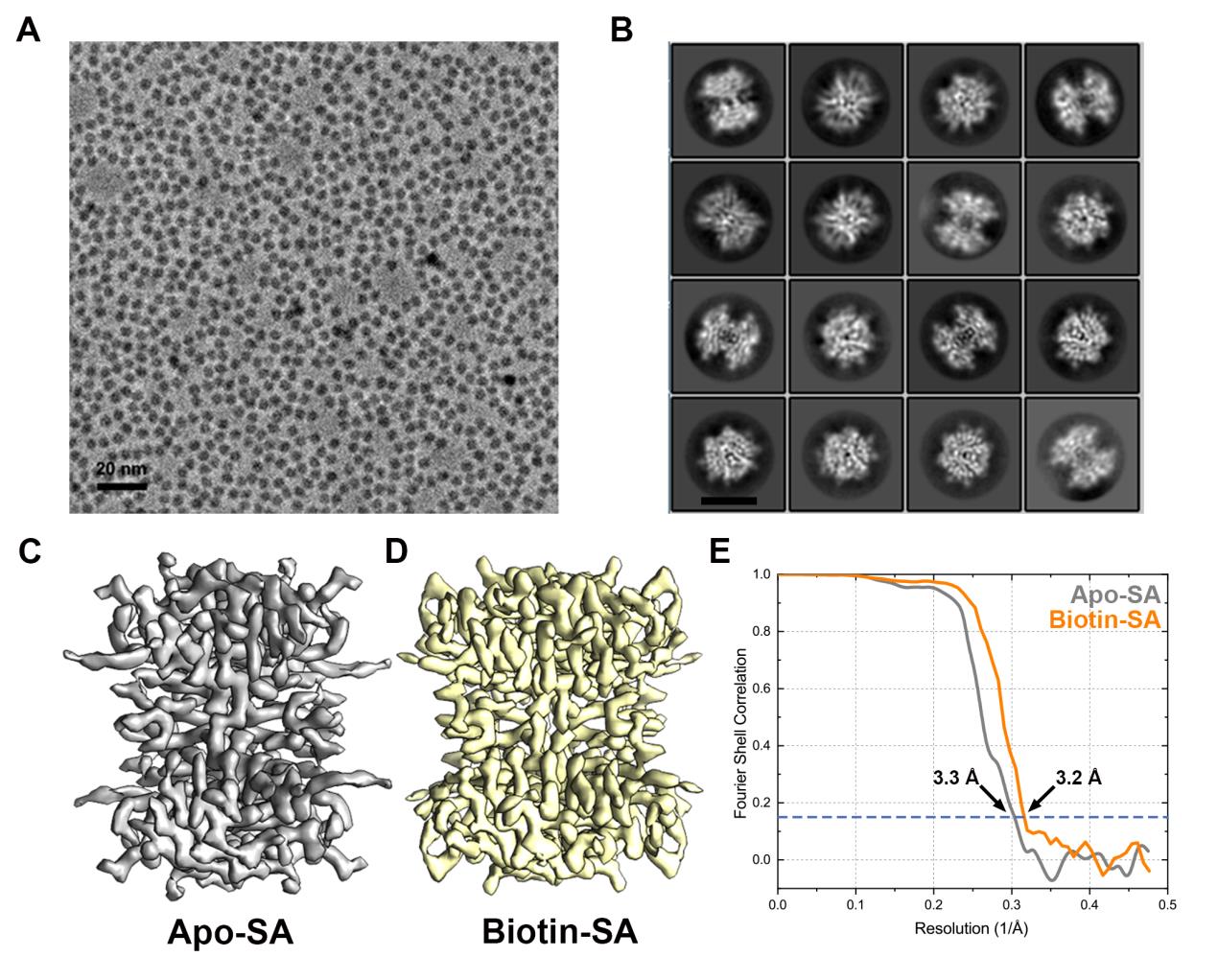
1) The fast development of single particle cryo-EM has made it more feasible to obtain the 3D structure of well-behaved macromolecules with a molecular weight higher than 300 kDa at ~3 Å resolution. However, it remains a challenge to obtain the high-resolution structures of molecules smaller than 200 kDa using single particle cryo-EM, mainly due to the low contrast of the molecules embedded in vitreous ice. In this work, we apply the Cs-corrector-VPP coupled cryo-EM to study the 52 kDa streptavidin (SA) protein supported on a thin layer of graphene and embedded in vitreous ice. We are able to solve both the apo-SA and biotin-bound SA structures at near-atomic resolution using single particle cryo-EM. We demonstrate that the method has the potential to determine the structures of molecules as small as 39 kDa. Furthermore, we find that avoiding the adsorption of proteins onto the air-water interface is critical to maintaining the high-resolution structural information.
Fan X, Wang J, Zhang X, Yang Z, Zhang JC, Zhao L, Peng HL, Lei JL*, Wang HW*. (2019) Single particle cryo-EM reconstruction of 52 kDa streptavidin at 3.2 Angstrom resolution. Nat. Commun., 10(1): 2386 (*co-corresponding authors)
2) Replication and transcription of influenza virus genome mainly depend on its RNA-dependent RNA polymerase (RdRP), composed of the PA, PB1, and PB2 subunits. Although extensively studied, the underlying mechanism of the RdRP complex is still unclear. Using single-particle cryo-electron microscopy, we have reconstructed the RdRP tetramer complex at 4.3 Å, highlighting the assembly and interfaces between monomers within the tetrameric structure.

Chang S, Sun D, Liang H, Wang J, Li J, Guo L, Wang X, Guan C, Boruah BM, Yuan L, Feng F, Yang M, Wang L, Wang Y, Wojdyla J, Li L, Wang J, Wang M, Cheng G, Wang HW*, Liu Y*. (2015) Cryo-EM Structure of Influenza Virus RNA Polymerase Complex at 4.3 Å Resolution. Mol. Cell, 57(5):925-935 (*co-corresponding authors)
3) Toll-like receptors (TLRs) have crucial roles in innate immunity, functioning as pattern-recognition receptors. TLR13 recognizes a conserved sequence from bacterial 23S rRNA and then triggers an immune response. In a collaboration work with Prof. Jiawei Wang and Jijie Chai at Tsinghua University, we developed a new set of approaches to solve the phasing problem of X-ray crystallography structural determination of the TLR13-RNA complex (300kDa as a dimer) using single particle cryo-EM reconstruction. My lab obtained the complex’s cryo-EM structure at 5 Å resolution and Prof. Jiawei Wang solved the crystal structure at 2.5 Å resolution using the EM map as initial phase information. This work was a very nice synergetic combination of X-ray crystallography with cryo-EM in structural biology and evealed important insights into innate immunity.

